Abstract
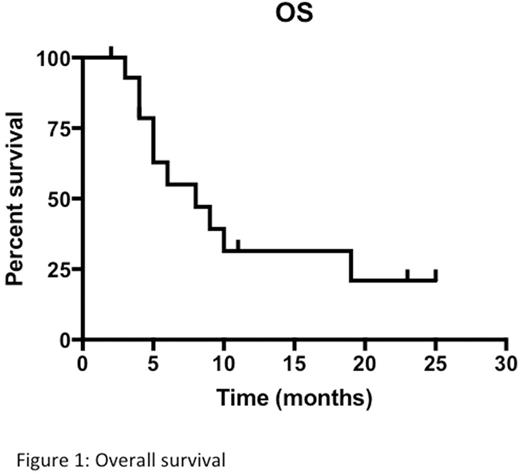
Background: Acute myeloid leukemia (AML) in elderly patients (pts) has a worse prognosis than in the younger because of age, comorbidities and frequent adverse biological characteristics, which cause ineffectiveness of standard treatments. Allogeneic stem cell transplantation is the treatment of choice in intermediate-high risk AML but it is not feasible in the majority of pts aged >65. It has been recently reported that allogeneic peripheral blood stem cells (PBSC), obtained with G-CSF stimulation from haploidentical donors and infused directly after standard induction chemotherapy (CT), without a conditioning regimen, significantly reduced the duration of post-treatment cytopenia and increased the complete remission (CR) rate and the 2-year disease-free survival in AML patients (Guo, Blood, 2011). The same micro-haplo stem cell infusion (mHaploSCI) procedure was effective as consolidation therapy in AML (Guo, JCO 2012). Aim of the study: We hypothesized that mHaploSCI after standard CT may be useful in elderly AML pts not candidate to allogeneic SCT. Herein we report the results of a multicenter prospective phase II study conducted to test safety and potential efficacy of mHaploSCI in elderly AML at intermediate/adverse ELN risk. Primary safety endpoint was grade IV extrahematologic toxicity and/or grade II-IV GVHD. Primary efficacy endpoint was the rate of continuous CR 12 months after diagnosis. The study has been carried out within the REL network and funded by Regional Council Resolutions of the Region Lombardy in Italy. Patients and methods: Eligible pts were registered at diagnosis and mHaploSCI was scheduled after the second CT cycle to allow time to screen the potential donor and to collect PBSC without delaying the start of induction CT. A minimum of 6x106 CD34+ cells/kg were collected and frozen without manipulation to allow two to three reinfusions of 2x106 haploidentical PBSC/kg after salvage, in pts with residual leukemia after first induction, and/or after each of two consolidation cycles in pts achieving CR. No immunosuppressive treatments was scheduled. Pts were managed with the usual supportive measures used in AML. Results: Of 18 pts registered, 3 were not evaluable because they never received mHaploSCI (asymptomatic myocardial infarction at screening, sepsis on day 18, and atrial flutter and cardiac failure on day 36 of induction). The characteristics of 15 evaluable pts were: median age 72 (range 66-75), M/F ratio 10/5, 2010 ELN risk: int-1: 4, int-2: 3, adverse: 7, NE: 1. Six pts had secondary or therapy-related AML. Induction consisted of classical 3+7 regimen (daunorubicin at 45mg/sqm/d). CR was achieved in 7 pts (47%), one obtained partial remission (PR) and 7 were treatment failures. Seven CR pts received the first mHaploSCI after consolidation with miniICE and 8 after salvage with MICE (1 PR) or MEC (7 refractory), according to protocol. Grade 1 rash developed on day 5 after the first mHaploSCI in 3 pts. CR was confirmed in 5 pts in CR after 3+7 (1 ongoing, 1 relapse) and was achieved in 3 of 8 pts after salvage. A second mHaploSCI was performed after consolidation with ID-AraC in the 8 CR pts. Four maintained CR (1 death of sepsis, 3 relapse). Only 1 of 3 pts who had obtained CR after salvage could receive a third mHaploSCI as per protocol. Hematologic recovery after mHaploSCI occurred after a median of 25 days (range 10-48) for neutrophils and of 27 days (range 17-60) for platelets. Overall there were no infusion reactions and no evidence of GVHD throughout the study. The presence of donor origin lymphocytes in mHaploSCI recipients was evaluated by short tandem repeat (STRs) testing in CD56+cells with a sensitivity of 2,5%. In 2 pts the transient presence of 5.34% and 3.33% alloPBL was documented at day 14 and 63 after mHaploSCI without relation to hematologic CR. Of 4 pts completing the protocol, 2 relapsed after 5 and 7 months and two are in continuous CR. At last follow up 5 pts are alive, 3 of them in CR. Median actuarial survival of the entire group is 7 months (range 1-25) (Figure 1). Conclusion: This prospective phase II study shows that adding mHaploSCI to standard CT in elderly pts was feasible and safe and did not cause any GVHD signals. However, mHaploSCI did not shorten the duration of post-CT cytopenia and its antileukemic efficacy appeared to be at best modest in a the setting of poor prognosis elderly AML.
Rossi: AbbVie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal